Drugmakers have paid out billions of dollars to patients who have claimed they have been harmed by drugs in one way or another. But what is a drugmaker's responsibility when a packaging problem results in an unplanned pregnancy and the consumer is looking for financial support for raising a child they weren't prepared to have? That is what more than 100 women intend to discern with suits against Endo Health Solutions ($ENDP) and Patheon.
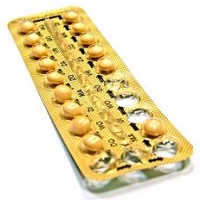 The litigation has been filed in state court in Pennsylvania on behalf of the 117 women, most of whom claim they became pregnant when the birth control pills they were taking were packaged in the wrong order, The Morning Call reports. Neither Endo nor Patheon would comment about the lawsuits, which the publication said were filed in state court after a federal judge in another jurisdiction declined to give the cases class action status.
The litigation has been filed in state court in Pennsylvania on behalf of the 117 women, most of whom claim they became pregnant when the birth control pills they were taking were packaged in the wrong order, The Morning Call reports. Neither Endo nor Patheon would comment about the lawsuits, which the publication said were filed in state court after a federal judge in another jurisdiction declined to give the cases class action status.
The suits track back to 2011 when Qualitest Pharmaceuticals, a unit of Endo, issued a recall of 3.2 million packages of contraceptives. The recall was set off when a Kansas City, MO, woman returned her birth control pills to the pharmacist after noticing that the blister pack had been rotated 180 degrees, reversing the weekly tablet orientation, The Morning Call reports, citing the lawsuit. Taking the right pill on the right day is crucial when taking hormonal contraceptives. The woman, Shanta Russell, was working two jobs and planning to go back to school at the time; she wasn't planning to get pregnant.
According to The Morning Call, a court order in the case said that of 117 women from 26 states represented by attorney Keith Bodoh, 113 became pregnant from the pill mix-up and 94 gave birth. Some had twins. At least one child was said to have been given up for adoption by a mother who was being deployed by the military, and one woman dropped out of law school and another out of nursing school after getting pregnant.
At least one other drugmaker has also faced paying for a part of parenthood after similar packaging missteps. Canadian generics maker Apotex was sued in 2013 by 45 women after it recalled about half a million packages of contraceptives when it was found some contained 14 active contraceptive pills and 14 placebos instead of the 21 active and 7 placebos that they should contain.
Novartis' ($NVS) Sandoz unit, Pfizer ($PFE), and Glenmark Pharmaceuticals have all had to recall contraceptives for similar packaging problems or other stumbles. In April, Pfizer acknowledged that about 100 women in Canada had gotten packages of expired birth control pills after a drug distributor accidentally shipped out the wrong products.
- read the story